CARBON is a valuable resource. Rather than wasting that to the atmosphere, it should be reused. Industry can use different forms of pure carbon in numerous ways.
There are good reasons to be unhappy when seeing flue gas rising from a fossil fuel power plant. Carbon dioxide (CO2) can make as much as 10−25 volume percent of the flue gas1. Every time we emit CO2 to the atmosphere, we speed up the climate change. In addition we waste a valuable resource, pure carbon, to the sky.
Carbon is almost everywhere. Carbon is the 15th most abundant element in the Earth’s crust. We human beings have been using that for different purposes as long as we have existed. We wouldn’t even exist without carbon because all life is based on that.
Carbon is a very versatile and safe material. Researchers keep on finding new applications for pure carbon all the time. Very many giant steps in technological development would not be possible without using pure carbon in novel ways.
Pure carbon has several allotropes, forms of a chemical element that occur in the same physical state. The different forms arise from the different ways atoms may be bonded together. For instance, both graphite and diamonds consists of pure carbon. Still graphite is one of the world’s softest materials and diamond is one of the hardest. This is caused by their distinct crystal structures2. The wide range of allotropes make pure carbon fit for very different uses.
Graphene is strong
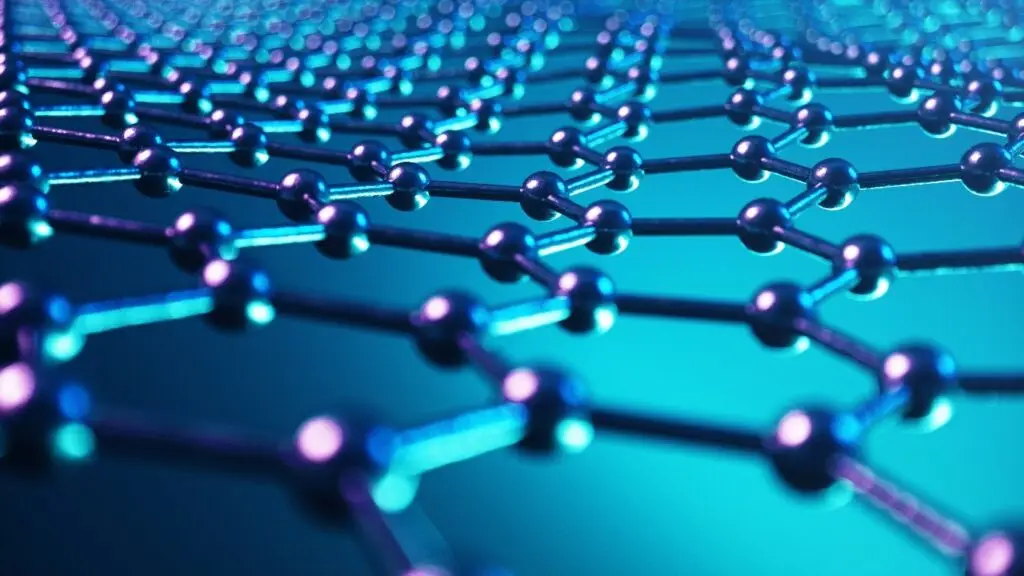
Graphene, the very basic carbon allotrope, is a flat sheet of strongly bonded carbon atoms in hexagonal cells. It is the basic structural element of other allotropes. It is about 100 times stronger than the strongest steel3. It conducts heat and electricity very efficiently and is nearly transparent4.
Because graphene is so strong, it can be used to enhance the strength of other materials. Even a small amount of graphene to plastics, metals or other materials can make these materials much stronger. If you incorporate graphene in a composite material, you can use other elements less in the material and reduce the weight without compromising with strength requirements. This is increasing all the time the use of graphene-based composite materials in airplanes, building materials and for instance in mobile applications.5
As the world’s most conductive material to heat, graphene is also a great material to make heat-spreading solutions, such as heat sinks or films used to dissipate heat. This could be useful in both microelectronics. For instance, it makes LED lighting more efficient and longer lasting.
As the world’s thinnest material, graphene is a very promising material to be used in batteries and supercapacitors. It may enable batteries and supercapacitors to store more energy and charge faster.
Graphene has a lot of other promising applications: anti-corrosion coatings and paints, efficient and precise sensors, faster and efficient electronics, flexible displays, efficient solar panels, faster DNA sequencing, drug delivery, and more.
Novel innovations with carbon nanotubes
Carbon nanotube, another allotrope, is a rolled graphene sheet with hemispherical ends. These tubes can be single-walled with a diameter of less than 1 nanometer or multi-walled, consisting of several concentrically interlinked nanotubes, with diameters reaching more than 100 nm.6
Apart from their electrical properties, which they inherit from graphene, carbon nanotubes also have unique thermal and mechanical properties that make them intriguing for the development of new materials. For instance, their mechanical tensile strength can be 400 times that of steel, their density is one sixth of that of steel and their thermal conductivity is better than that of diamond.
All these properties make carbon nanotubes ideal candidates for electronic devices, chemical/electrochemical and biosensors, transistors, electron field emitters, lithium-ion batteries, white light sources, hydrogen storage cells, cathode ray tubes, electrostatic discharge and electrical-shielding applications. They can even be used in very basic objects like sports equipment.

Graphite stands high temperatures
When graphene sheets stack above each other, we have graphite. The sheets stack to create volume, but the vertical bonds between the sheets are very weak making graphite so soft. Graphite is widely used as a lubrificant because it reacts with atmospheric water vapor to deposit a thin film over any adjacent surfaces and reduces the friction between them. It forms a suspension in oil and lowers friction between two moving parts.7
Graphite is also a common refractory material because it withstands high temperatures without changing chemically. It is used in manufacturing processes ranging from steel and glass making to iron processing. It is also an asbestos substitute in automobile brake linings.
Wider use of batteries have also increased the use of graphite. Lithium-ion batteries have a lithium cathode and a graphite anode. As the battery charges, positively charged lithium ions in the electrolyte – a lithium salt solution – accumulate around the graphite anode.
Diamonds are a driller’s best friends
In a diamond, the carbon atoms are arranged tetrahedrally. Each carbon atom is attached to four other carbon atoms. It is a strong, rigid three-dimensional structure that results in an infinite network of atoms. This accounts for diamond’s hardness, extraordinary strength and durability and gives diamond a higher density than graphite. Because of its tetrahedral structure, diamond also shows a great resistance to compression.8
Because of their hardness, diamonds are extremely useful when used to cut, grind, or drill other materials. Therefore, many cutting blades or drills have small diamonds included on the tips and edges.9
Fullerene helps carry drugs

In contrast to graphite and diamond, fullerenes are spherical molecules and are soluble in a variety of organic solvents, an important requirement for chemical manipulations.10 It has several medical applications because of its features. For instance, fullerene is able to fit inside the hydrophobic cavity of HIV proteases, inhibiting the access of substrates to the catalytic site of enzyme. It can be used as radical scavenger and antioxidant. Fullerenes can be used to cleave DNA. In addition, fullerenes have been used as a carrier for gene and drug delivery systems. Also they are used for serum protein profiling as MELDI material for biomarker discovery.11
Fullerenes has remarkable electrochemical features and electrocatalysis. Using this material for modification provides an increase in the peak currents in electro analysis due to the chemical stability of fullerene. Using fullerene in the construction of biosensors improves the stability and potential window.12
You drive on tires with carbon black
Pure carbon has even more forms that extend its use to additional applications.

Carbon black is a form of paracrystalline carbon that has a high surface-area-to-volume ratio. The most important application area of carbon black are tires. Around 9 million tonnes were processed in tires worldwide in 2017. Rubber products for industry and construction ranked second. Carbon Black is processed in a wide range of products that are used in the chemical industry, mechanical engineering, the construction industry as well as the electrical and electronics industry. Products include conveyor belts, roll coverings, tubes, profiles, seals, cables, moldings, and roofing foils. Some black carbon is also used as a black pigment in printing inks and plastics.13
Activated carbon cleans the mess
Activated carbon is a form of carbon processed to have small, low-volume pores that increase the surface area available for adsorption or chemical reactions. Unlike carbon black, it is not very conductive. The surface-area-to-volume ratio of activated carbon is higher than that of carbon black.14
Activated carbon is used in methane and hydrogen storage, air purification, solvent recovery, decaffeination, gold purification, metal extraction, water purification, medicine, sewage treatment, air filters in gas masks and respirators, filters in compressed air, teeth whitening, production of hydrogen chloride in dark and many other applications.15
Sources:
1) https://en.wikipedia.org/wiki/Flue_gas
2) https://www.thoughtco.com/allotrope-definition-in-chemistry-606370
3) https://www.semanticscholar.org/paper/Measurement-of-the-elastic-properties-and-intrinsic-Lee-Wei/7ad70a564c4c8898f61eddbcecf9e90f71e9d4b6
4) http://www.graphene-battery.net/graphene-properties.htm
5) https://www.graphene-info.com/graphene-applications
6) https://www.nanowerk.com/nanotechnology/introduction/introduction_to_nanotechnology_22.php
7) https://sciencing.com/uses-graphite-5670130.html
8) https://www.scientificamerican.com/article/how-can-graphite-and-diam/
9) http://scienceviews.com/geology/diamond.htmlactivated carbon
10) https://www.sciencedirect.com/topics/engineering/carbon-allotrope
11) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2676811/
12) https://www.sciencedirect.com/science/article/pii/B978012816144900002X
13) https://www.ceresana.com/en/market-studies/chemicals/carbon-black/
14) https://www.differencebetween.com/difference-between-carbon-black-and-activated-carbon/
15) https://en.wikipedia.org/wiki/Activated_carbon