Technology
Hycamite is a pioneer of affordable decarbonization

Energy-efficient, low-carbon hydrogen production
87% less
Hycamite’s methane-splitting technology energy requirement versus electrolysis.
90% less
Hycamite’s methane-splitting technology carbon footprint versus steam methane reforming (SMR).
Disruptive technology
Hycamite’s low-carbon technology captures carbon in a solid, high-quality form, offering opportunities for carbon capture, use and storage (CCUS).
We break down methane using heat and our proprietary, recyclable catalysts. Hycamite has developed several families of catalysts that, compared to other methane pyrolysis processes, work at lower temperatures and increase the quality of the solid carbon obtained from methane-splitting.
This disruptive and IP-protected process, developed by Hycamite, builds on more than twenty years of research from the University of Oulu, Finland.

Efficient hydrogen production
Low-emissions technology saves energy. Hycamite’s methane-splitting technology requires 87% less energy compared to hydrogen production via electrolysis. By utilizing novel Hycamite catalysts, we significantly reduce the required process temperature and, thus, the amount of energy used. The total energy needed is 6 to 10 kWh/kg H2, of which a considerable part can be heat. This is very low compared to electrolysis, which uses approximately 50 to 55 kWh/kg H2.
Since more than half of Hycamite’s energy needs is used for heat, clients’ waste heat can be utilized for hydrogen production.
50+
New Hycamite methane-splitting plants by 2030
Low carbon footprint
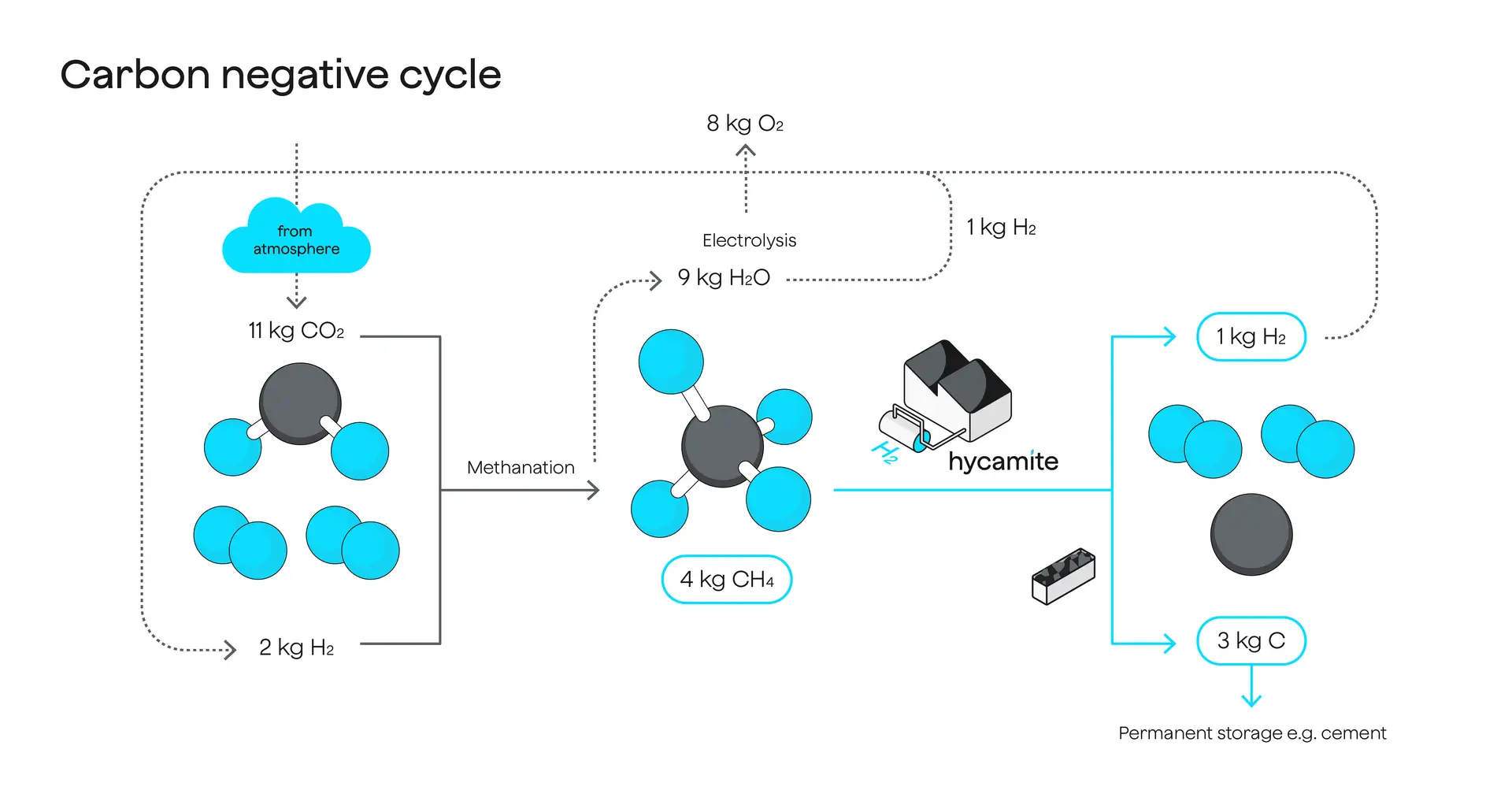
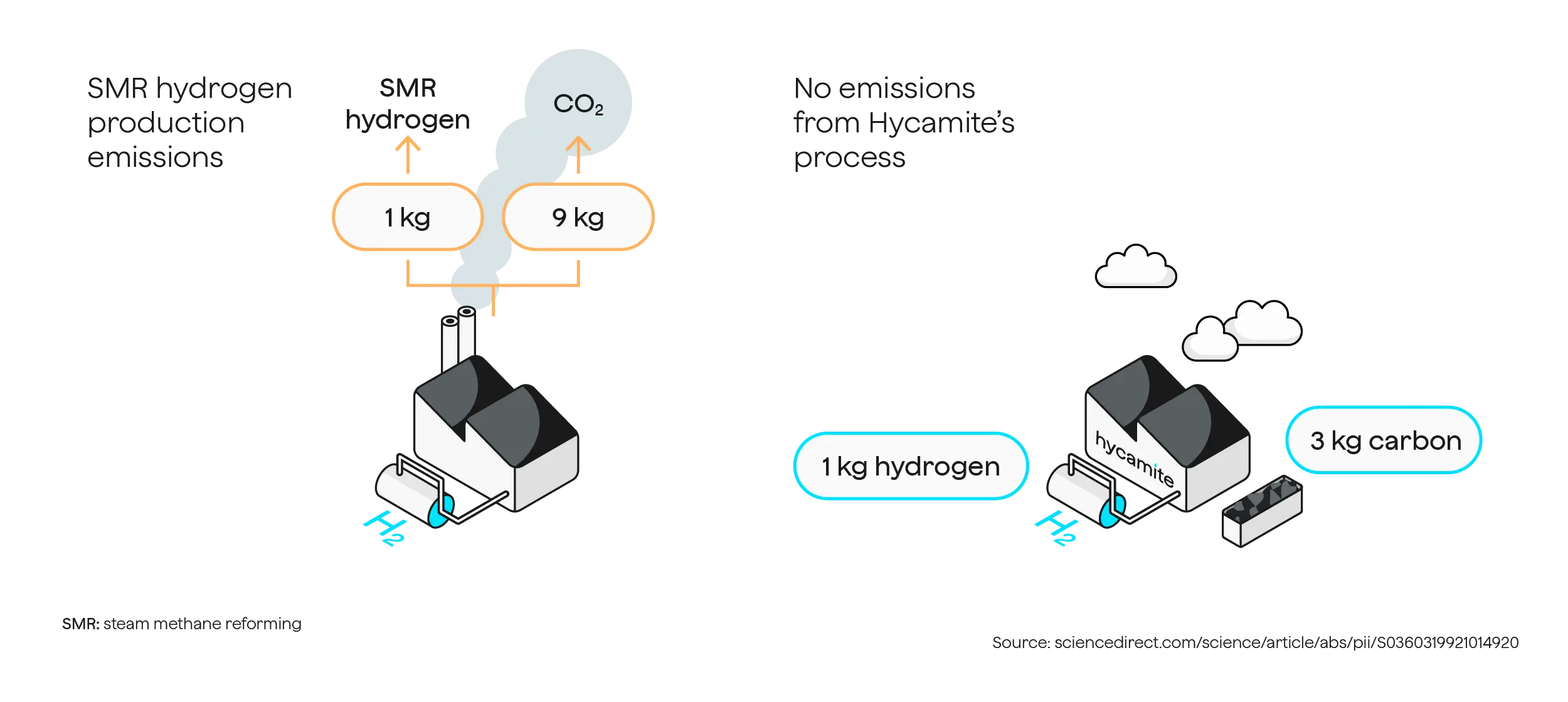
The HYCAMITE™ process is entirely oxygen-free. After the methane molecule has been split, the carbon is immediately collected in a solid form. Due to the absence of oxygen in the process, no carbon dioxide can form, thereby preventing any leakage into the atmosphere.
Hycamite’s methane-splitting technology, as assessed by the German research institute FfE, boasts a life-cycle carbon footprint below 1 kg CO2e/kg H2 in Finland. When biogas is utilized as a source of methane, the end products act as a carbon sink, ultimately removing carbon dioxide from the atmosphere. In contrast, steam methane reforming (SMR) produces hydrogen with a higher CO2 footprint of 9 to 20 kg CO2e/kg H2.
The HYCAMITE™ process operates without oxygen, which allows for immediate solid carbon collection after splitting methane. This prevents the formation of carbon dioxide and accidental leaks into the atmosphere. While some may refer to our method as turquoise hydrogen, we prefer to focus on the carbon intensity of the hydrogen we produce. This is due to the lack of clear definitions for hydrogen colors and their relevance to actual life-cycle emissions.

Using methane solves many problems
The HYCAMITE™ process utilizes methane as a feedstock due to its wide availability, scalability, and ease of storage and transportation using existing infrastructure. Whether obtained from natural gas, biomethane, or synthetic sources, even petrochemical by-products can be used responsibly. We prioritize suppliers with low emissions.
Our methane-splitting plants can be easily installed near customers, eliminating the need for costly infrastructure. Feedstock is transported via existing methane pipelines and processed on-site, saving time and expenses.