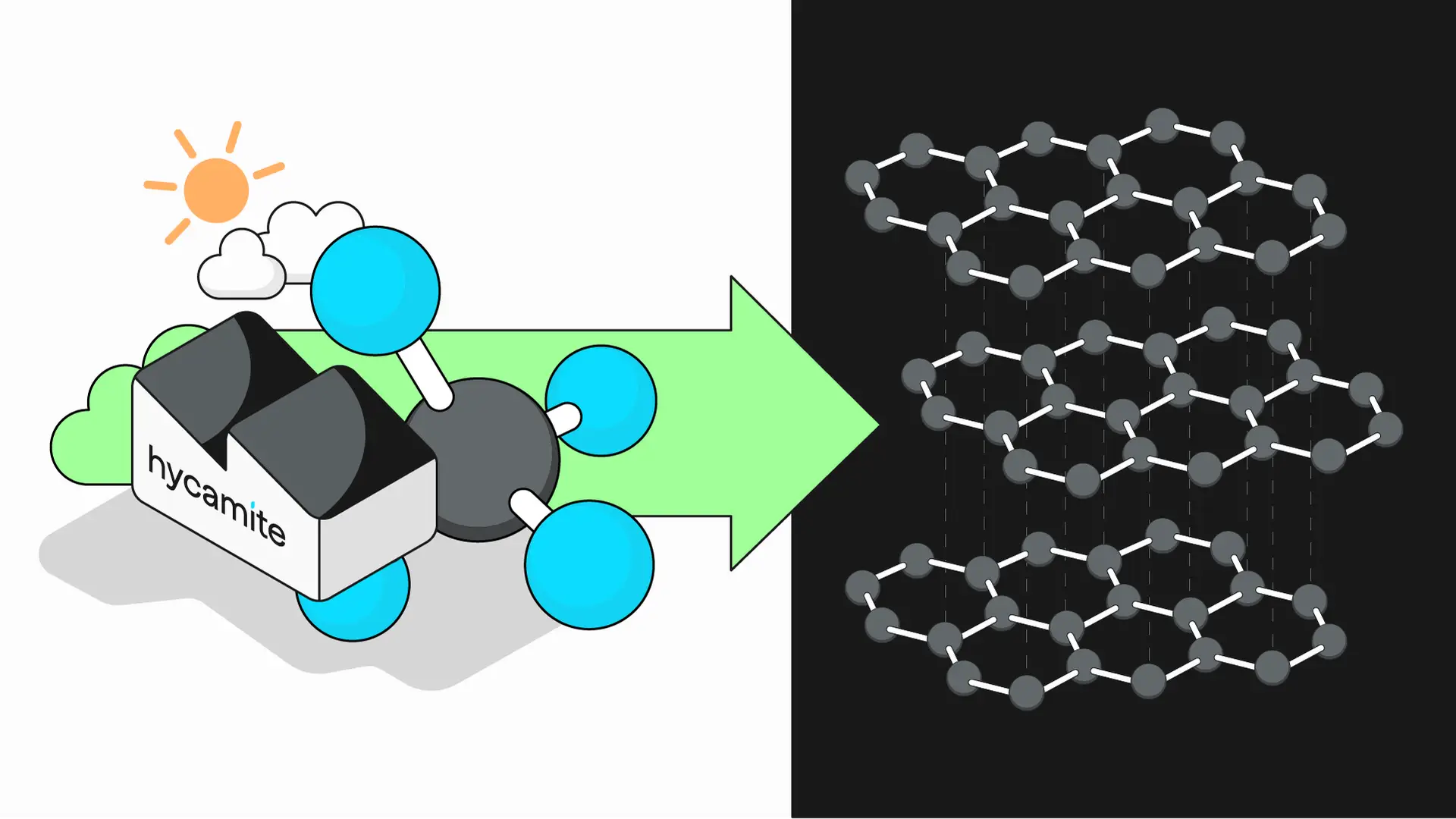
Synthetic graphite has so far been produced through a complex process of baking petroleum coke at very high temperatures. The traditional graphite precursor is mostly petroleum needle coke that is derived from by-products of the oil refinement. The damage to the environment caused by graphite production has been wide and varied — much more than just extensive atmospheric emissions — as covered in my previous article about graphite.
When discussing with experts at several companies, it has become clear how strongly sustainability concerns are now driving efforts to reduce carbon footprints and develop sustainable practices. Customers in different industries are increasingly targeting the reduction of carbon dioxide (CO2).
At the same time, the global demand for carbon products is increasing, driven by the energy, automotive and electronics sectors, among others. The carbon and graphite market is projected to grow from 15 billion in 2022 to almost USD 25 billion by 2030, with a compound annual growth rate of 6.3%.
As the number of industries and the need for more sustainably made graphite grows, the current risks regarding the origin of supply must decrease. The need for reliable and scalable sources of carbon materials, including graphite, is critical due to geopolitical concerns and supply chain vulnerabilities.
Unlimited quantities of graphite have never been closer
Our researchers have worked long and hard to solve the problem of graphite production and client proximity. I am very proud of my team and honored to say that we now have a proof of concept for producing synthetic graphite with significantly lower emissions and less energy. As our capacity increases, we can produce high-value graphite in unlimited quantities, close to industrial customers worldwide and with a very low carbon footprint.
The new method uses novel methane-splitting technology to provide low-carbon (turquoise) hydrogen and sustainable carbon products for demanding industrial applications. The technology decomposes large volumes of methane into its component elements — hydrogen and carbon — while avoiding the release of greenhouse gases (GHGs) into the atmosphere. Furthermore, Hycamite’s catalysts are sustainable as they are recyclable and can be produced from industrial side streams. Our researchers have reached our graphite development goal thanks to advances in proprietary catalysts and process technology.
Low carbon footprint, high-value product
According to a life cycle assessment (LCA), using Hycamite’s carbon products can significantly lower GHG emissions when producing numerous carbon-containing products. The results show Hycamite’s carbon footprint to be below 1 kg CO2e/kg C for Hycamite’s carbon as it comes out from the reactor. Senior Sustainability Specialist Salla-Mari Marttinen’s latest article provides more details about calculating the LCA.
Methane pyrolysis reduces CO2 emissions in the upstream process primarily in two ways. It replaces high-carbon feedstocks with natural gas or biogas and decreases energy consumption by ~5.3 kWh by reducing the number of process steps. With highly efficient catalysts, methane splitting reduces energy consumption for graphite precursor production by ~3.5 to 4.5 kWh.
The production method also helps solve other challenges. For example, there are no mining, environmental or ethical concerns as long as the methane provided has low upstream emissions.
When using methane as the feedstock, we can also produce graphite locally, close to the end client, as much as the client needs. This offers security of supply for these critical materials. Reliable local deliveries also significantly lower the transportation’s CO2 footprint.
Electric vehicles drive the demand
Our new technology enables us to produce a range of carbon products. Several of our high-value carbon products are electrically conductive, making them ideal for supercapacitors, electronics, electric cables, additives in polymers and building elements made with composite materials or concrete. By using the range of its catalyst families and changing process parameters, we can even tailor high-value carbon products to individual customer needs.
However, there is clearly a very high demand for graphite – mainly due to electric vehicles (EVs). Future growth in graphite, both synthetic and natural, is driven almost completely by battery production. Exponential growth in EV sales is transforming the automotive sector faster than currently predicted, with EVs set to dominate global car sales by the end of the decade. Graphite is currently at the forefront of demand as it is used as an anode in nearly all commercialized lithium-ion batteries (Li-ion or LIBs) today.
Graphite quality improvement continues
Our graphite development is progressing rapidly, achieving graphitization levels similar to battery-grade graphite.
We are dedicated to continuous improvement and are actively optimizing our graphite through battery testing. We are excited to partner with anode manufacturers to co-develop and customize our graphite to meet the specific needs of different battery chemistries. Together, we aim to create tailored solutions that drive innovation and efficiency in the battery industry
Our new facility to open in September
We are already taking significant steps to scale our production. Hycamite is currently building its new Customer Sample Facility (CSF) in Kokkola, Finland, with an anticipated opening in the coming fall. The facility is intended to demonstrate the viability of the new methane-splitting technology, and it will be the largest methane pyrolysis facility in Europe.
Once the project is fully completed, CSF’s nominal capacity will reach 2,000 tons of hydrogen and 6,000 tons of high-quality carbon per year. This amount of carbon would be enough for about 100 000 EV batteries. When liquefied natural gas is used, CSF’s decarbonization capacity can be up to 18,000 tons of CO2 per year, and carbon removals can also occur with biomethane.
This is just the beginning. Follow us for new facility announcements and updates on our decarbonization journey.